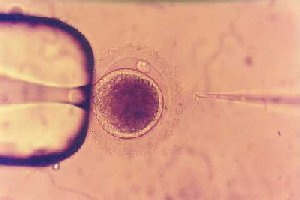
| Intracytoplasmic sperm
injection: a novel treatment for all forms of male factor
infertility. Fertil. Steril. 63: 1231~1240.
Porcu, E., R. Fabbri, R. Seracchioli, P. M.
Ciotti, O. Magrini, L. Savelli, T. Ghi and C. Flamigni. 1997. Intracytoplasmic sperm
injection of cryopreserved human oocytes. In: In Vitro Fertilization and Assisted
Reproduction (V. Gomel and P. C. K. Leung, eds.), Monduzzi Editore S.P.A., Bologna, Italy.
Pp 1153~1157.
Pursel, V. G., R. J. Wall, C. E. Rexroad, Jr., R.
E. Hammer and R. L. Brinster. 1985. A rapid whole-mount staining procedure for nuclei of
mammalian embryos. Theriogenology 24(6): 687-691.
Silber, S. J., A. C. Van Steirteghem, J. Liu, Z.
Nay, H. Tournaye and P. Devroey. 1995. High fertilization and pregnancy rate after
intracytoplasmic sperm injection with spermatozoa obtained from testicle biopsy. Human
Reprod. 10: 148~152.
Tesarik, J., C. Mendoza and J. Testart. 1995.
Viable embryos from injection of round spermatids into oocytes. New Engl. J. Med. 333:
525.
Tesarik, J., F. Rolet, C. Brami, E. Sedbon, J.
Thorel, C. Tibi and A. Thebault. 1996. Spermatid injection into human oocytes. II.
Clinical application in the treatment of infertility due to non-obstructive azoospermia.
Human Reprod. 11: 780~783.
Tsubamoto, H., A. Hasegawa, M. Inoue, N. Yamasaki
and K. Koyama. 1996. Binding of recombinant pig zona pellucida protein 1 (ZP1) to
acrosome-reacted spermatozoa. J. Reprod. Fertil., Suppl. 50: 63-67.
Vanderzwalmen, P., G. Bertin, B. Lejeune, M.
Nijs, B. Vandamme and R. Schoysman. 1996. Two essential steps for a successful
intracytoplasmic sperm injection: injection of immobilized spermatozoa after rupture of
the oolema. Human Reprod. 11(3): 540~547.
Van Steirteghem, A. C., Z. Nagay, H. Joris, J.
Liu, C. Staessen, J. Smitz, A. Wisanto and P. Devroey. 1993. High fertilization and
implantation rates after intracytoplasmic sperm injection. Human Reprod. 8(7): 1061~1066.
Wassarman, P. M. 1988. Zona pellucida
glycoproteins. Ann. Rev. Biochem. 57: 415-442.
Wu, M. C., Y. S. Luoh and J. F. Liou. 1997.
Comparisons on survival rates of porcine embryos cryopreserved by slow cooling for various
breeds and cell stages. J. Taiwan Livestock Res. 30(1): 97-109.
Yurewicz, E. C., B. A. Pack and A. G. Sacco.
1991. Isolation, composition, and biological activity of sugar chains of porcine zona
pellucida 55K glycoproteins. Mol. Reprod. Develop. 30: 126-134.
|